Discovering genomic and developmental mechanisms that underlie sensory innovations critical to adaptive diversification
Aug 26, 2014
 Photo by Stephen Rossiter
Photo by Stephen Rossiter
Publications
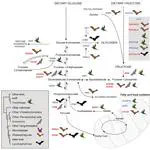
While evolvability of genes and traits may promote specialization during species diversification, how ecology subsequently restricts such variation remains unclear. Chemosensation requires animals to decipher a complex chemical background to locate fitness-related resources, and thus the underlying genomic architecture and morphology must cope with constant exposure to a changing odorant landscape; detecting adaptation amidst extensive chemosensory diversity is an open challenge. In phyllostomid bats, an ecologically diverse clade that evolved plant-visiting from an insectivorous ancestor, the evolution of novel food detection mechanisms is suggested to be a key innovation, as plant-visiting species rely strongly on olfaction, supplementarily using echolocation. If this is true, exceptional variation in underlying olfactory genes and phenotypes may have preceded dietary diversification. We compared olfactory receptor (OR) genes sequenced from olfactory epithelium transcriptomes and olfactory epithelium surface area of bats with differing diets. Surprisingly, although OR evolution rates were quite variable and generally high, they are largely independent of diet. Olfactory epithelial surface area, however, is relatively larger in plant-visiting bats and there is an inverse relationship between OR evolution rates and surface area. Relatively larger surface areas suggest greater reliance on olfactory detection and stronger constraint on maintaining an already diverse OR repertoire. Instead of the typical case in which specialization and elaboration are coupled with rapid diversification of associated genes, here the relevant genes are already evolving so quickly that increased reliance on smell has led to stabilizing selection, presumably to maintain the ability to consistently discriminate among specific odorants — a potential ecological constraint on sensory evolution.
Laurel R. Yohe,
Matteo Fabbri,
Daniela Lee,
Kalina T.J. Davies,
Thomas P. Yohe,
Miluska K.R. Sanchez,
Edgardo M. Rengifo,
Ronald P. Hall,
Gregory L. Mutumi,
Brandon P. Hedrick,
Alexa Sadier,
Nancy B. Simmons,
Karen E. Sears,
Elizabeth R. Dumont,
Stephen J. Rossiter,
Bhart-Anjan Bullar,
Liliana M. Dávalos
In most vertebrates, the demand for glucose as the primary substrate for cellular respiration is met by the breakdown of complex …
Joshua Potter,
Rosie Drinkwater,
Kalina T.J. Davies,
Nicolas Nesi,
Marisa C.W. Lim,
Laurel R. Yohe,
Hai Chi,
Xiaoqing Zhang,
Ilya Levantis,
Burton K. Lim,
Christopher C. Witt,
Georgia Tsagkogeorga,
Mario dos Reis,
Yang Liu,
William Furey,
Matthew J. Whitley,
Dunja Aksentijevic,
Liliana M. Dávalos,
Stephen J. Rossiter
Comprising more than 1,400 species, bats possess adaptations unique among mammals including powered flight, unexpected longevity, and …
Diana D. Moreno-Santillán,
Tanya M. Lama,
Yocelyn Gutiérrez Guerrero,
Alexis M. Brown,
Paul Donat,
Huabin Zhao,
Stephen Rossiter,
Laurel R. Yohe,
Joshua Potter,
Emma C. Teeling,
Sonja Vernes,
Kalina T.J. Davies,
Eugene Myers,
Graham M. Hughes,
Zixia Huang,
Federico G. Hoffmann,
Angelique P. Corthals,
David Ray,
Liliana M. Dávalos
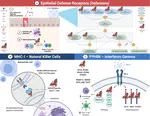
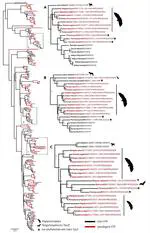
Bats possess extraordinary adaptations, including flight, echolocation, extreme longevity and unique immunity. High-quality genomes are crucial for understanding the molecular basis and evolution of these traits. Here we incorporated long-read sequencing and state-of-the-art scaffolding protocols1 to generate, to our knowledge, the first reference-quality genomes of six bat species (Rhinolophus ferrumequinum, Rousettus aegyptiacus, Phyllostomus discolor, Myotis myotis, Pipistrellus kuhlii and Molossus molossus). We integrated gene projections from our ‘Tool to infer Orthologs from Genome Alignments’ (TOGA) software with de novo and homology gene predictions as well as short- and long-read transcriptomics to generate highly complete gene annotations. To resolve the phylogenetic position of bats within Laurasiatheria, we applied several phylogenetic methods to comprehensive sets of orthologous protein-coding and noncoding regions of the genome, and identified a basal origin for bats within Scrotifera. Our genome-wide screens revealed positive selection on hearing-related genes in the ancestral branch of bats, which is indicative of laryngeal echolocation being an ancestral trait in this clade. We found selection and loss of immunity-related genes (including pro-inflammatory NF-κB regulators) and expansions of anti-viral APOBEC3 genes, which highlights molecular mechanisms that may contribute to the exceptional immunity of bats. Genomic integrations of diverse viruses provide a genomic record of historical tolerance to viral infection in bats. Finally, we found and experimentally validated bat-specific variation in microRNAs, which may regulate bat-specific gene-expression programs. Our reference-quality bat genomes provide the resources required to uncover and validate the genomic basis of adaptations of bats, and stimulate new avenues of research that are directly relevant to human health and disease1.
David Jebb,
Zixia Huang,
Martin Pippel,
Graham M. Hughes,
Ksenia Lavrichenko,
Paolo Devanna,
Sylke Winkler,
Lars S. Jermiin,
Emilia C. Skirmuntt,
Aris Katzourakis,
Lucy Burkitt-Gray,
David A. Ray,
Kevin A. M. Sullivan,
Juliana G. Roscito,
Bogdan M. Kirilenko,
Liliana M. Dávalos,
Angelique P. Corthals,
Megan L. Power,
Gareth Jones,
Roger D. Ransome,
Dina Dechmann,
Andrea G. Locatelli,
Sebastien J. Puechmaille,
Olivier Fedrigo,
Erich D. Jarvis,
Mark S. Springer,
Michael Hiller,
Sonja C. Vernes,
Eugene W. Myers,
Emma C. Teeling